 |
 |
|
|
Newsletter #11 - November 23, 2001
This memo summarizes the essential details of the Point of Care Connectivity Industry Consortium (CIC) MEDICA 2001 Meeting, held November 22 on the Roche boat "MS Britannia" in Düsseldorf. Approximately forty attendees from throughout Europe joined representatives of the CIC and European standardization organizations for this important meeting on Europe-specific issues involving point-of-care connectivity.
In this issue:
- Executive summary
- Presentations
- Participants
- Remaining timeline, milestones
- Next steps europe
- Attendees
The Connectivity Industry Consortium (CIC) held its fourth major briefing meeting for Point-of-Care organizations outside of the United States on November 27, 2001 in Düsseldorf, Germany. The briefing was held in conjunction with the MEDICA 2001 conference. Thirty-nine individuals representing twenty-eight international point-of-care (POC) organizations attended this meeting.
During last year's MEDICA briefing, the CIC launched a European Round Table (ERT) effort to ensure that POC suppliers and users in Europe are informed about and have an opportunity to contribute to international POC standards. One objective of this year's Medica briefing was for the ERT to report on its progress and solicit further input from international POC users.
After reviewing the ERT's progress, attendees expressed great interest in being notified of and involved in future ERT activities. To facilitate this communication, the CIC agreed to setup a mailing list to connect all parties interested in European standardization issues surrounding POC connectivity.
Horst Merkle, Roche Diagnostics and CIC Vice President, opened the meeting. Jeff Perry, Walker Informatics and CIC Chief Technical Officer, then presented the CIC's Sunset plans and current status. Next, Thomas Norgall, Fraunhofer Institute, presented a status report and summary of strategic direction for the CIC's European Round Table effort. Andrew St. John, IFCC Scientific Division, updated attendees on the progress toward drafting a 'whitepaper' on point-of-care connectivity issues. Finally, Sean Field, Roche Diagnostics, presented some results of a Roche survey of Laboratory Information Systems in today's point-of-care testing environment.
Although the CIC organization is sunsetting and will cease to exist early in 2002, the Briefing's attendees made it clear that there is still a great deal of work to be done to educate European point-of-care users and vendors about how existing standards can provide cost-effective multi-vendor connectivity. To address this issue, the CIC's sunset plans will ensure that the work begun by the ERT initiative can continue beyond the sunset of the CIC organization. The CIC will dedicate funds and resources to continue the European outreach initiative begun by the ERT.
The following presentations may be viewed on the CIC website at www.poccic.org/Meetings/Medica2001.
- Horst Merkle - Roche Diagnostics, CIC Vice-President: Opening, Objectives (Action Items) and Agenda
- Jeff Perry - Walker Informatics, CIC Chief Technical Officer: CIC Sunset and Current Standard Status
- Thomas Norgall - Fraunhofer-Institut für Integrierte Schaltungen IIS, Erlangen, CIC Europe Chairperson, CIC Europe - Status Quo of the Strategic Direction of the European CIC Initiative
- Andrew St. Johns - IFCC Scientific Division - Creation of a European White Paper
- Sean Field - Roche Diagnostics, Laboratory Information Systems in today's POCT environment
- Horst Merkle - Roche Diagnostics, CIC Vice-President: Discussion, Q&A
- Thomas Norgall - Fraunhofer-Institut für Integrierte Schaltungen IIS, Erlangen, CIC Europe Chairperson, Next Steps Europe and Closing
Approximately forty individuals representing twenty-eight different organizations attended the briefing, as summarized in the following two tables.
| COUNTRY | ATTENDEES |
|---|---|
| Australia | 1 |
| Denmark | 1 |
| Finland | 3 |
| Germany | 18 |
| Netherlands | 2 |
| Spain | 1 |
| Swiss | 5 |
| UK | 3 |
| USA | 5 |
| Total | 39 |
Participants represented the in-vitro diagnostics industry, information technology vendors, point-of-care users, healthcare consultancies, and other point-of-care related organizations. The following table summarizes the attendance by the type of organization represented.
| PARTICIPANTS | ORGANIZATIONS | |
|---|---|---|
| Diagnostics | 15 | 5 |
| Information Technology | 3 | 3 |
| User | 10 | 10 |
| Consultant | 7 | 7 |
| Standardizations Org. | 3 | 2 |
| Others | 1 | 1 |
| Total | 39 | 28 |
A detailed list of all attendees is contained in the last section of these minutes.
The CIC accomplished several major milestones since the previous year's Medica briefing. In March, the CIC vendor members and the Provider Review Committee ratified the connectivity specification. Six weeks later, the NCCLS published this specification as a 'proposed level' standard, named AUTO6-P. Comments and suggestions were collected over a six month review period.
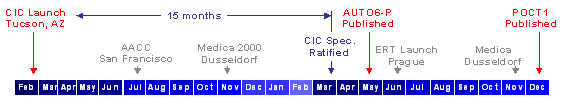
All outstanding issues will be resolved before the end of 2001, and the NCCLS anticipates publishing a final 'approved level' standard, named POCT1 in the first week of January 2002.
Several action items were proposed and agreed to during the briefing. A mailing list has been established to facilitate communication between individuals interested in European issues surrounding standardization of point-of-care connectivity. If you are interested in being added to this mailing list, or would like to distribute information to the list, please contact the CIC European Round Table General Secretary, Cristina Rode-Schubert, at the following e-mail address: christina.rode-schubert@MBE.unisg.ch
Also, Thomas Norgall will investigate the possibility of involving the Fraunhofer institute in developing prototype or demonstration implementations of the CIC protocols. A plan that would culminate in hosting a CIC multi-vendor-demonstration of these prototypes in the Fraunhofer booth at Medica 2003 would be of particular interest.
Thirty-nine individuals from twenty-eight organizations registered to attend the CIC Medica 2002 Briefing. The following table provides a detailed list of attendees and further members.
| ATTENDEE | ORGANIZATION | COUNTRY |
| A. Dyson | Telemedicine System Development Medgate AG | Swiss |
| Andreas Boos | Philips Medizinsysteme GmbH | Germany |
| Andreas Staubert | InterComponentWare Germany | Germany |
| Andrew St. John | Consulting | Australia |
| Andrzej Knafel | Roche Instrument Center | Swiss |
| Ch. Rode-Schubert | CHROSCH-Consulting | Germany |
| Chris Morgan | The Technology Partnership plc | UK |
| Chris Schofield | Roche Diagnostics Germany | Germany |
| Christian Baer | BSG Unternehmensberatung | Swiss |
| Dirk Boecker | Pelikan Technologies USA | USA |
| Doug Hirst | Bradford Royal Infirmary UK | UK |
| Emery Stephans | EAC USA | USA |
| Gabriella von Flittner | Orion Diagnostica Fin | Finland |
| Georg Hoffmann | Trillium GmbH | Germany |
| Gert Fendesak | Roche Diagnostics | Germany |
| H.R.Fischer | Möbius Projektkoordinator, Kantonsspital Basel | Swiss |
| Hans-Bernd Bludau | University Hospital Heidelberg | Germany |
| Harald Schlebusch | University of Tübingen | Germany |
| Harm Overbeek | Vital Sientific | Netherlands |
| Heiko Ziervogel | Agilent Technologies | Germany |
| Heinz Glismann | Roche Diagnostics | Germany |
| Horst Merkle | Roche Diagnostics | Germany |
| Jan L. S. Dols | Academic Medical Center / University of Amserdam | Netherlands |
| Jeff DuBois | NCCLS / nova biomedical | USA |
| Jeff Perry | Walker Informatics | USA |
| Joachim Dudeck | University of GiessenMember of GMDS advisory board | Germany |
| Josef Bogyim | PHILIPS Medical Systems | Germany |
| Klaus Kjoller | Radiometer | Denmark |
| Lou Dunka | NCCLS / Lifescan | USA |
| Lutz Heinemann | Profil GmbH / Institut für Stoffwechselforschung | Germany |
| Michael Brenner | PHILIPS Medical Systems | Germany |
| Ossi Hiltunen | Orion Diagnostica | Finland |
| Paul Mundill | Orion Diagnostica | Finland |
| Ricardo Ruiz | Trends in Technology, S.L.&Universidad Politecnica de Madrid | Spain |
| S. Reichlin | Projektmanager Telemedizin, Medgate AG | Swiss |
| Thomas Norgall | Fraunhofer-Institute IIS-A, Erlangen | Germany |
| Tito Bacarese-Hamilton | gsk Consultancy Services | UK |
| Volker Weidinger | Executive Manager Medical Networks (MN) / "DeutschlandMed" | Germany |
| Zlotan Takacs | Philips Medical Systems | Germany |
| comments: webmaster | |
| |
|
© 2003 European Initiative |
|