 |
 |
|
|
Newsletter #7 - July 9, 2000
In this issue:
Attendees at the American Association of Clinical Chemistry (AACC) annual meeting in San Francisco, July 23-27, will have plenty of opportunities to see and hear about the CIC.
- CIC Exhibit Booth #542
You are cordially invited to visit the CIC's booth, number 542, on the Moscone Center Exhibit floor. Stop by the booth to meet Consortium representatives. Members of the CIC's technical teams will be available to answer questions about the Consortium's standards, and to explain the benefits the industry will realize from these standardization efforts. In addition, several CIC members will be demonstrating prototype systems that use the draft CIC standards to communicate simple patient test results.
Special thanks to the AACC for sponsoring this booth, and also to the many CIC members who volunteered to staff the booth.
- POCD Industrial Liaison Committee Meeting
LifeScan/OCD will sponsor the Point of Care Division Industrial Liaison Committee meeting on Tuesday, July 25. At this meeting, the CIC will present its current organizational status and technical progress. This meeting will be held in Golden Gate Hall B, from 7:00 to 9:30AM, and is open to all conference attendees and CIC members.
- CIC Technical Working Meeting
The CIC's second Technical Working meeting will take place on Thursday, July 27 from 7:30AM to 5:00PM. Bayer Diagnostics has generously offered to host this meeting at the Sheraton Palace Hotel, located a few blocks away from the Moscone Center. At this meeting, the CIC technical teams will review the first draft of the Device and EDI patient test result standards. The teams will also begin work on extensions to these draft message sets that will support Ordering and QA/QC processes. This meeting is open to all.
- CIC Members' Booths
Please visit the following CIC members' booths to learn more about how these organizations plan to take advantage of the CIC standards.
Organization Booth # Abaxis
320
Abbott Laboratories
605
Agilent Technologies
3719
AVL Scientific
3025
Bayer Diagnostics
3401
BD
3601
COLA
239
HemoCue
622
i-STAT
605
ITC
1819
LifeScan/OCD
691, 1305
Medical Automation Systems
521
Pharmacia & Upjohn
3619
Radiometer Medical
3611
Roche Diagnostics
520
Sigma Diagnostics
919
- CIC Member Preregistration
If you belong to a CIC member organization and would like to participate in one of the CIC sponsored events, please complete the preregistration web form. Within two days of completing the form, you will receive a confirmation message, and will be registered to receive additional pre-meeting information.
By the AACC meeting, the CIC will have completed 5 months of its planned one-year lifetime.
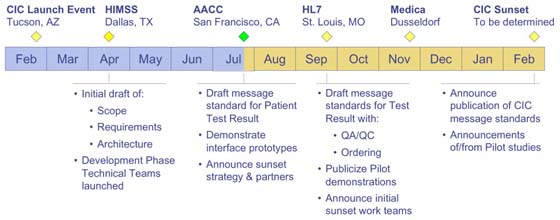
The Consortium is on-target to meet the deliverables specified for the AACC milestone. At this meeting, the Consortium will conduct a technical review of the draft standards for Patient Test Results. Also, four member organization (Agilent Technologies, LifeScan, Medical Automation Systems and Roche Diagnostics) are scheduled to demonstrate prototype implementations of the CIC standards. Finally, good progress has been made toward establishing partnerships with chartered standards organizations that are interested in continuing development of the CIC's work after the Consortium's sunset.
A significant amount of work remains to be done to extend the standard message sets to accommodate ordering and QA/QC workflow. If the Consortium continues to make progress at the rate it has accomplished to date, all the remaining milestones will be met on schedule.
The Consortium is pleased to welcome several new members. As of July 10, 2000, the CIC boasts 33 member organizations (11 Core Vendors, 10 Core Providers, 4 Individual Providers, 18 Supporting Vendors).
| Core Vendor | Core Provider | |
|---|---|---|
Abbott Laboratories |
Banner Health System |
|
Agilent Technologies |
Bradford Royal Infirmary |
|
AVL Scientific |
Geisinger Medical System |
|
Bayer Diagnostics |
Johns Hopkins Medical Institutions |
|
BD |
Kaiser Permanente |
|
Instrumentation Laboratory |
Mayo Clinic |
|
LifeScan/OCD |
The Mount Sinai Hospital |
|
Medical Automation Systems |
Profil GmbH |
|
Radiometer Medical |
St. Vincent Mercy Medical Center |
|
Roche Diagnostics |
University of Iowa Healthcare |
|
Sunquest |
||
| Individual Provider | Supporting Vendor | |
Maurice Green, Ph.D. |
Abaxis |
|
Neil Halpern, MD |
Avocet Medical |
|
LTC Forrest Kneisel |
Cerner |
|
Gerald Kost, MD, Ph.D. |
Citation Computer Systems |
|
Petrie Rainey, MD, Ph.D. |
GE Marquette Medical Systems |
|
HemoCue |
||
HemoSense |
||
i-STAT |
||
| Liaison Organizations | ITC |
|
AACC |
InterComponentWare |
|
COLA |
Medtronic |
|
IFCC Scientific Division |
Motorola |
|
Pharmacia & Upjohn |
||
SMS |
||
STC Technologies |
||
Sigma Diagnostics |
||
Telcor |
||
VIA Medical |
If your organization would like to join the Connectivity Industry Consortium, please contact the CIC's President, Suzanne Cross.
Best regards,
Jeff Perry
CIC Vice-President, Chief Technical Officer
Representing: Agilent Technologies
You have received this newsletter because you are on the mailing list for the Point of Care Connectivity Industry Consortium. If you would like to be removed from this mailing list, please send an e-mail to info@med.labs.agilent.com with the subject "Remove CIC". If you would like to be added to the mailing list, please fill out the web form.
| comments: webmaster | |
| |
|
© 2003 European Initiative |
|