 |
 |
|
|
Newsletter #8 - September 18, 2000
In this issue:
- Technical Meeting
The CIC held its third major technical meeting in San Francisco on July 27, 2000. Bayer Diagnostics sponsored the meeting, which was attended by 57 individuals representing 28 organizations from 15 states and 4 countries.
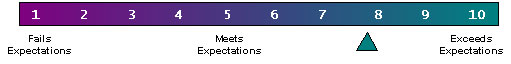
Participants Organizations IS/IT Vendors 105POC/IVD Vendors 3414POC Customers/users 96Others 43Totals 5728Meeting participants reviewed the Consortium's technical progress. Attendees also developed 8 week work plans to address several remaining technical issues: messages to support QA/QC, and ordering workflows. Finally, participants evaluated and critiqued the CIC's progress to date. When asked to rate the Consortium's progress relative to their expectations, attendees responded positively with an average score of 7.89 on a scale of one to ten (high: 11, low: 5).
You may view a detailed summary of this meeting on the CIC website.
- Exhibit Booth
In order to increase the CIC's public visibility, the AACC sponsored booth #542 in the conference exhibit hall on behalf of the Consortium.
 Members of the CIC's technical teams staffed the booth to answer questions about the
Consortium's standards, and to explain the benefits the industry will realize from these standardization
efforts.
Members of the CIC's technical teams staffed the booth to answer questions about the
Consortium's standards, and to explain the benefits the industry will realize from these standardization
efforts.The booth's posters described the CIC's history, objectives, membership, and first pilot project. In addition, several CIC members demonstrated prototype systems that use the draft CIC standards to communicate simple patient test results (see following story).
The Consortium would like to thank the AACC for sponsoring this booth, and also the many CIC members who volunteered to staff the booth.
- POCT Division Industrial Liaison Meeting
The 2000 AACC POCT Division Industrial Liaison meeting kicked off with the auspicious news that the Point-of-Care Division had become
 the largest single division of the AACC.
the largest single division of the AACC.The meeting's attendees heard Ms. Cathy Burzik, President, Ortho Clinical Diagnostics give the keynote address. She compared the CIC’s one-year mission (and good progress to date) with her experience with the seven-year struggle in imaging to establish industry-wide standards.
Ms. Suzanne Cross (CIC President) and Mr. Jeff Perry (CIC C.T.O.) followed with reports on the CIC's overall and technical progress, respectively. Mr. Mike Higgins (Agilent Technologies) described progress toward the first CIC pilot implementation: a home coagulation demonstration being developed by Agilent Technologies, Avocet Medical, Kaiser-Permanente, Medical Automation Systems, and Sunquest Information Systems. Finally, Dr. Ellis Jacobs and Dr. Jim Nichols presented the Provider Review Committee's review of the Consortium's progress.
The meeting summary, authored by Mr. Emery Stephans, Chair of the Industrial Liaison Committee, may be viewed on the CIC website.
The CIC's next public meeting will be held in conjunction with the Medica 2000 Conference in Dusseldorf, Germany. Roche Diagnostics will host this meeting, which will likely take place on Thursday, November 24.
The objectives of this meeting are:
- to present the CIC's progress and status to the international point-of-care user community
- to solicit user feedback on the CIC's proposed standards
- to enlist international user's help with ongoing review and advocacy of the CIC standards
Best regards,
Jeff Perry
CIC Vice-President, Chief Technical Officer
Representing: Agilent Technologies
| comments: webmaster | |
| |
|
© 2003 European Initiative |
|